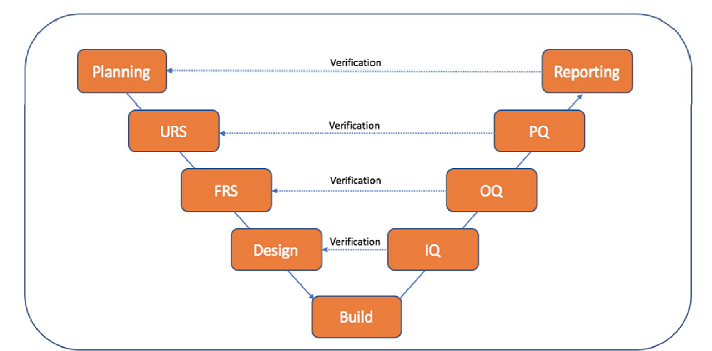
Drug and medical device manufacturing in the modern world relies increasingly on computerized systems. Being highly regulated industries, ensuring quality and accuracy of data is paramount; and thus, Computer/Computerized System Validation (CSV) is necessary to assure that critical processes are functioning properly.
Bionic Technologies is dedicated to helping companies address the requirements in 21 CFR Part 11 Electronic Records and Electronic Signatures, and EU Annex 11 and Computerized Systems.
Bionic Technologies is providing the validation services with the following of regulatory norms. Our experienced validation team will document the consistency, quality, and accuracy of your systems, methods, and processes. We understand the different validation requirements for GMP, GLP, and GCP systems and the best ways to ensure compliance with FDA regulations. We can follow your validation procedures or assist your company in creating an appropriate process.
The software development life cycle (SDLC) is a series of phases that provide a common understanding of the software building process. How the software will be realized and developed from the business understanding and requirements elicitation phase to convert these business ideas and requirements into functions and features until its usage and operation to achieve the business needs. A good software engineer should have enough knowledge on how to choose the SDLC model based on the project context and the business requirements.Therefore, it may be required to choose the right SDLC model according to the specific concerns and requirements of the project to ensure its success
Types of Software developing life cycles (SDLC):